APT and AMT Difference

The difference of ammonium paratungstate (APT) and ammonium metatungstate (AMT) firstly lays in their molecular structure, as the picture bellow:
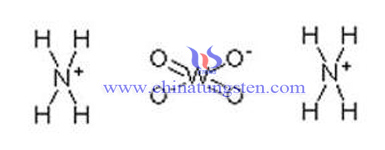
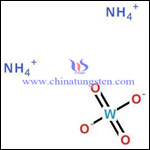
Difference of molecular formula:
1. The molecular formula of ammonium metatungstate is (NH4)6H2W12O40.nH2O or (NH4)6H2W12O40.XH2O or (NH4)6H2W12O40
2. The molecular formula of APT maybe different according to the different crystallization conditions, such as:
3(NH4)2O-7WO3-6H2O ;
5(NH4)2-12WO3-5H2O;
5(NH4)2O-12WO3-5H2O;
5(NH4)2O-12WO3-11H2O
Difference of solubility:
1. AMT has a good solubility of 303.99/100g in water under 20℃, and the solution is quite stable, but can not soluble in alcohol;
2. Ammonium paratungstate is slightly soluble in water with less than 2% of total weight dissolved at 20℃, and also insoluble in alcohol.
Difference of manufacture:
The biggest difference of AMT and APT is that APT is the raw material for preparing AMT, and the method of APT thermal degradation is the typical process of solid phase transformation method for manufacturing AMT.
Difference of application:
1. Ammonium metatungstate can be used for preparing metal tungsten powder, alloy steel and tungsten compound, and also used in the nuclear shielding materials, corrosion inhibitors ect.;
2. Ammonium paratungstate is mainly used in the manufacture of tungsten trioxide or blue tungsten oxide and thus to prepare tungsten powder, also it is used ad laboratory reagents for generating high purity tungstic acid, tungsten powder or tungsten carbide and water - absorbing gel.





